🛡️ Protect Yourself and Others from Unsafe Laser Devices
Every consumer has the right to receive safe and properly regulated laser hair removal treatments. Unfortunately, many laser hair removal businesses in Canada are using machines that are not certified, unlicensed, or outright counterfeit. If you spot something suspicious, you can report it directly to Health Canada through their official complaint system.
This guide walks you through the process — with examples, simplified explanations, and visual walkthroughs — so anyone can file a report (no technical knowledge required).
Last updated: October 13, 2024
Why Certification Matters: Safe Lasers Deliver Safer Results
Not every machine meets Canada's safety standards — but spotting a certified one can be easy with the right info. This guide will help you identify and report devices that don't meet regulations.
In This Guide:
- 🚩 When Should You Report a Device?
- 🕵️♀️ Confirm the Machine Falls Under Canadian Medical Device Regulations
- 📝 Step 0: Start the Process
- 🔍 Step 1: Description of Problem
- 🧾 Step 2: Device Details (if known)
- 👤 Step 3: About You
- ✅ Step 4: Consent
- 📤 Step 5: Submit Your Problem
- 🙌 Why Your Report Matters
- ❓ Still Have Questions?
- 🧠 Final Thoughts: You Have the Right to Speak Up
🚩 When Should You Report a Device?
You should file a report if you suspect the machine is fake, unlicensed, or unsafe, especially when:
- ❌ There are no Health Canada, CSA, SPE-3000, or IEC 60601 stickers on the device
- ⚠️ The machine looks unbranded, off-market, or oddly labeled
- 🧐 The brand isn't well-known (e.g. unknown "SHR" machines)
- ✈️ Staff mention they bought it from AliExpress, overseas, or secondhand
- ❌ They say it's "FDA-approved" (which does not apply in Canada)
- 💰 The price for treatments is suspiciously low (e.g. full body for $100–$150) compared to legitimate pricing
- 😟 You were concerned about the safety or professionalism during your visit
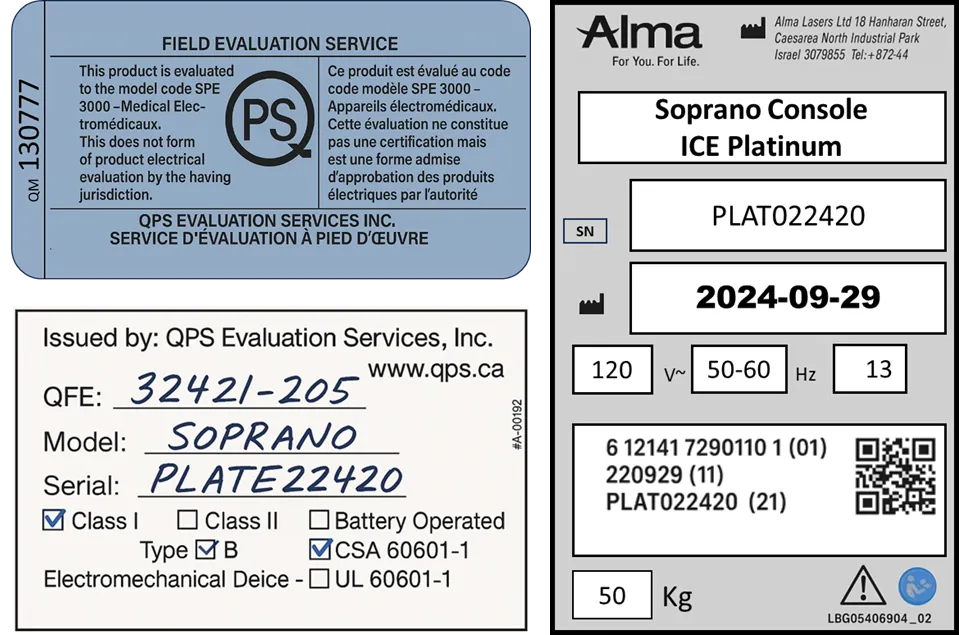
🕵️♀️ Confirm the Machine Falls Under Canadian Medical Device Regulations
Every legitimate laser hair removal machine in Canada must meet specific regulatory requirements. When examining a device:
- Look for three key certification labels on the back of the machine that tell you:
- Who manufactured the machine (with model and serial number)
- When it was built
- Certification that it passed Canadian medical safety standards
- Health Canada classifies most laser hair removal systems as Class I and II medical devices, which means:
- They must be licensed by Health Canada
- They must meet CSA or equivalent certification standards like IEC 60601-1 or SPE-3000
If any of these certification labels are missing, the device may not be compliant with Canadian regulations.
📄 View Health Canada's Medical Device Regulations
🔍 See our detailed guide on what legitimate certification labels look like
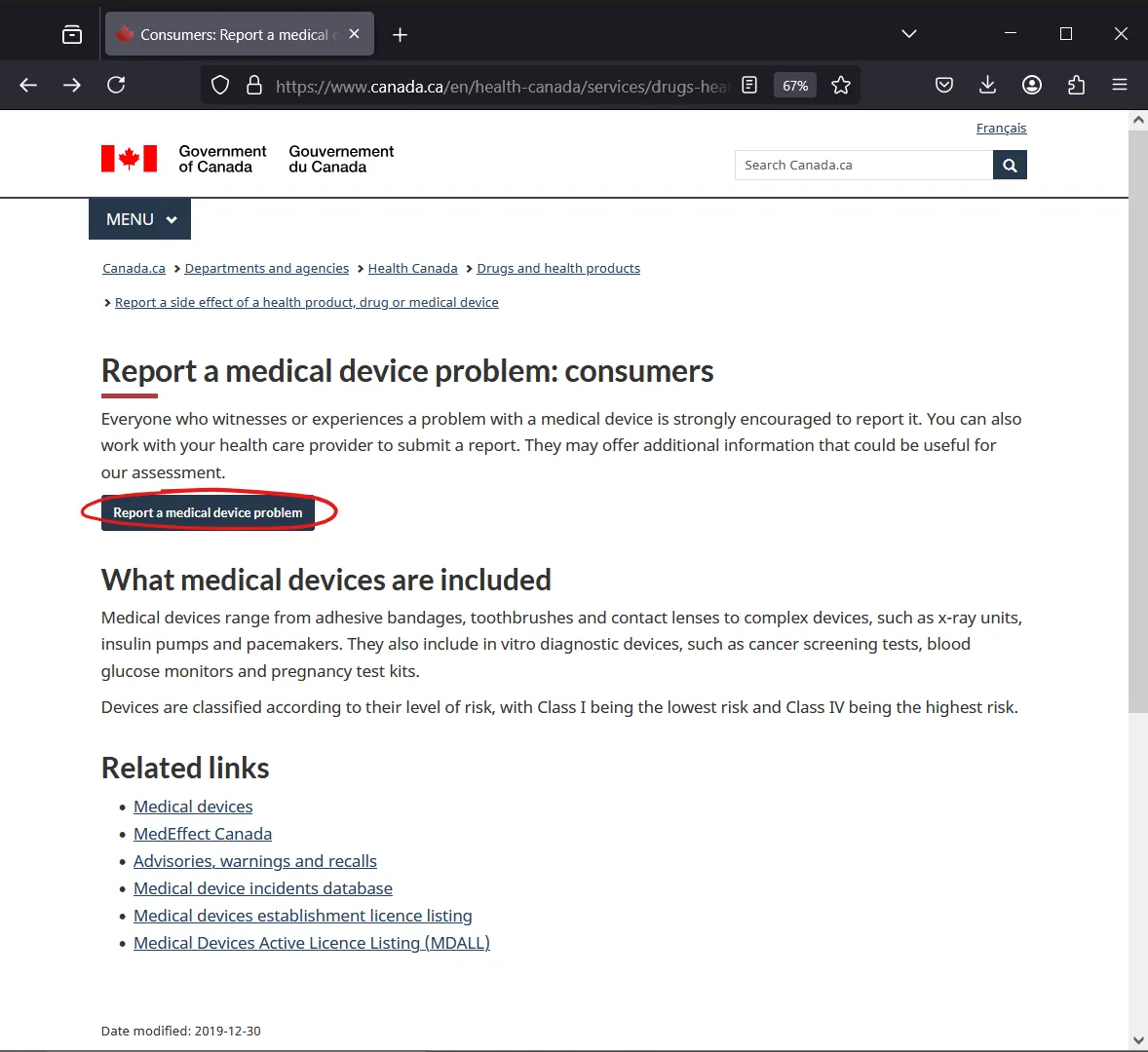
📝 Step 0: Start the Process
Visit the official Health Canada reporting page for consumers:
Click the blue button: "Report a medical device problem"
You'll be taken to the form titled: Consumer Medical Device Report Form
📷 Visual Walkthrough
Starting Point
Choose "Consumer" > Medical Devices
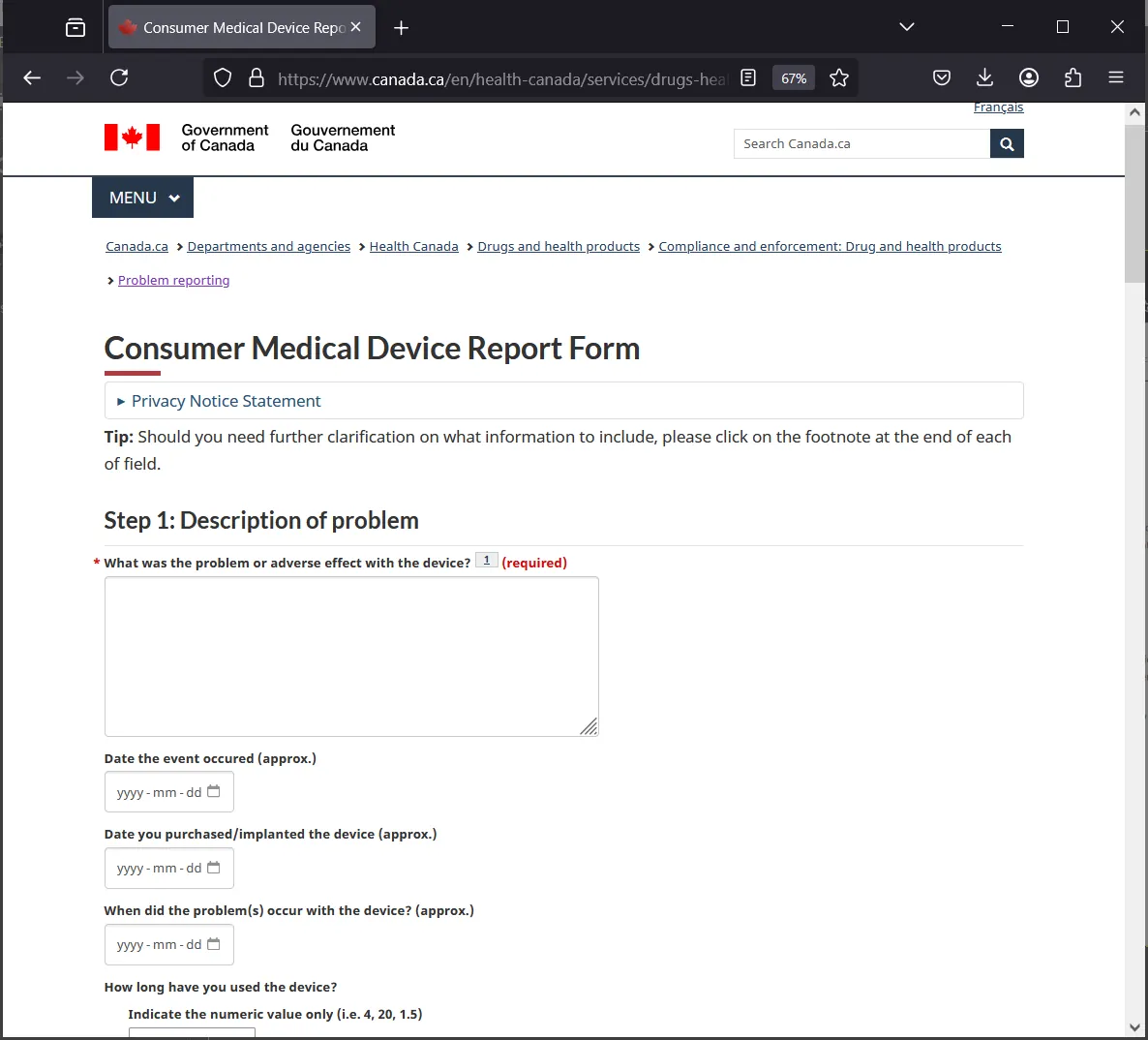
Form Interface
What the form looks like when it opens
🔍 Step 1: Description of Problem
🧩 1.1 Describe What You Saw
In your own words, describe what seemed wrong. You don't need to be an expert — just be clear and honest.
✅ You can mention things like:
- The machine had no Health Canada sticker
- There were no visible CSA, SPE-3000, or IEC 60601 labels
- The brand didn't sound familiar or official
- Staff said things like "we bought it overseas" or "it's FDA-approved"
- It looked suspicious or unsafe
💡 Not sure how to spot a real vs fake laser device?
🧠 Read our Blog: How to Identify a Certified vs. Fake Laser Hair Removal Machine in Canada
We walk you through official markings and what to avoid.
📍 1.2 Explain Where You Saw the Machine
You don't need to know who made the device or where it was bought. Instead, describe it like this:
- "I saw the machine during my appointment at [clinic name] in [city]"
- "The technician told me it was a 'new diode laser from China'"
- "It didn't have any certification stickers — just a logo that said 'BeautyMax SHR'"
- "They were offering full-body treatments for less than $50"
Did You Know?
According to Health Canada, more than 50% of reported laser machine injuries were caused by uncertified or off-market devices. Reporting makes a real difference in protecting consumers.
🧾 Step 2: Device Details (if known)
This section is optional — just fill in what you can. Health Canada can still take action even if some details are missing.
🔎 2.1 Device Type or Brand
Write what you saw on the machine:
- "Soprano Ice"
- "Alma Harmony XL"
- "BeautyMax SHR"
- "Diode Laser with no branding"
You can also include other types such as:
Soprano Titanium, Candela GentleLASE, Lumenis, Primelase, Elite IQ, or Venus Epileve.
🧩 2.2 Missing Licence or Safety Labels
This is key info. If the machine looked off, say it directly:
- "I did not see any certification or approval stickers."
- "The device looked generic with no brand or ID."
💡 You can also upload any pictures or screenshots.
🧠 You can report more than one suspicious machine in the same form using the "Add more devices" dropdown.
🔬 2.3 Model / Catalogue / Expiry (if available)
If you took a picture of the label or nameplate, include:
- Model/Catalogue Number
- Expiry Date (sometimes labeled with an hourglass symbol)
- Lot or Batch Number (look for "LOT" or a serial code)
Important Tip for Expiry Date
As a client, you likely won't know the exact expiry date of the device. If the form requires this information and the day is unknown, please select the last day of that month.
For example, if you only know it expires in March 2023, select March 31, 2023.
👤 Step 3: About You
Add basic contact information so Health Canada can follow up:
- First + Last Name (optional)
- Email (required)
- Phone number (optional)
- City, Province (optional)
Privacy Note
Health Canada keeps your personal information confidential. They need your contact details only to follow up if they have additional questions about your report.
Why Giving Consent Matters
When you allow Health Canada to share your report with manufacturers and regulatory partners, it helps them identify patterns of non-compliance across multiple reports. This can lead to faster action against unsafe devices in the market.
Want to Report Anonymously?
Only your email address is mandatory for submission. If you prefer to remain anonymous, you can leave the name and phone fields blank and still submit your report. Health Canada will only use your email if they need additional information.
✅ Step 4: Consent
You'll be asked:
- Can Health Canada share this report (without your personal info)?
- Can they contact you if needed?
We strongly recommend answering Yes to both — it helps speed up investigations.
🔒 Health Canada follows the Privacy Act and protects your personal information while sharing necessary details with regulatory partners.
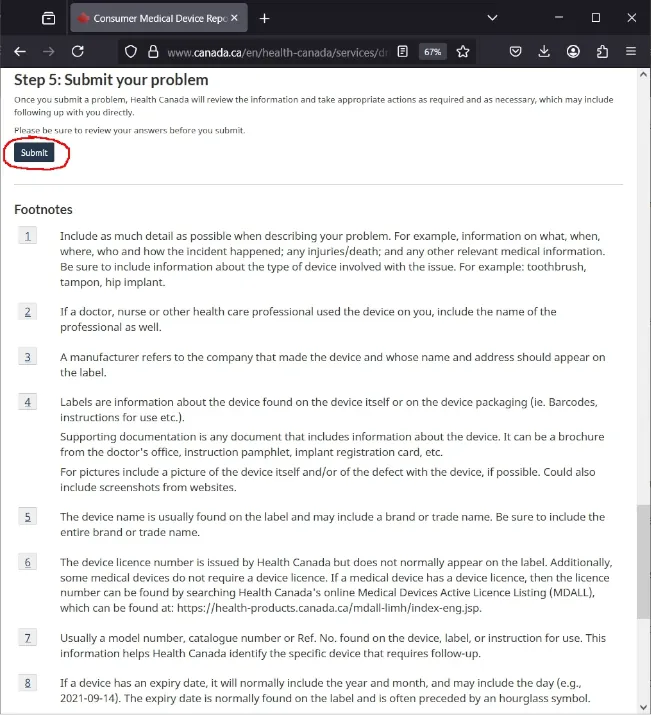
📤 Step 5: Submit Your Problem
Once all your information is filled out, just scroll down and click Submit.
Health Canada will review your complaint and may contact you for more details.
What Happens Next?
After submitting your report, Health Canada will review it and determine what action is needed. They may ask for additional information or evidence if necessary. You'll typically receive a confirmation email that your report was received.
🙌 Why Your Report Matters
Every submission helps Canadian authorities:
- Investigate illegal and unsafe equipment
- Educate and penalize non-compliant businesses
- Protect future clients
- ✅ And ensure fair business practices in the aesthetics industry
"I went to a spa that advertised the same laser technology as Bonita, but my skin was burned during treatment. When I asked to see their certification, they kept making excuses. I reported them to Health Canada and learned their machine was actually a knockoff. My report might prevent someone else from being hurt." — Sarah K., Former Client
❓ Still Have Questions?
If you're unsure how to describe the issue, spot a fake machine, or complete a specific step:
📩 Contact us directly or explore our growing list of educational blogs.
🧠 Want to learn more? Check out our FAQ page for more information or these related resources:
🧠 Final Thoughts: You Have the Right to Speak Up
Laser clinics in Canada are legally responsible for using licensed equipment. If you notice something off — no sticker, suspicious brand, evasive answers — say something.
At Bonita Laser, we proudly operate certified Soprano machines by Alma, fully inspected and licensed for use in Ontario. We believe every Canadian client deserves safe, effective treatment from certified technicians.